BENDU-381®
What Causes Allergies?
Allergies can be caused by any substance (an allergen) that the body’s immune system sees as a potential threat—things like pollen, pet dander, or smoke. Allergens lead to allergic reactions.

What are the Symptoms of Allergies?
Allergic reactions depend on the individual—they can vary in severity, vary with exposure, and vary over time. Sometimes they affect the whole body, or just a part, and sometimes they can be life-threatening.
Mild symptoms include: runny nose, itchy nose, sneezing, red/itchy eyes, watery eyes, asthma, rash, swelling, etc. Severe symptoms include: difficulty breathing, vomiting, low blood pressure, etc.
Globally, the frequency of the condition allergic rhinitis—also known as hay fever—is increasing. Allergic rhinitis is induced by allergens such as house ticks, pollen, fungus, animal dander, and exacerbated by pollution, food additives, and stress.

Allergic rhinitis presents recurring and long-lasting symptoms, such as chronic runny nose, sneezing, and congestion, which can make patients feel physically and emotionally exhausted in daily life and work. Typical treatments for allergic rhinitis include allergen avoidance, medication with anti-histamines and steroids, immune therapy, and, in severe cases, surgery. However, medicinal avenues can cause severe adverse effects with prolonged use, risking exposure to adverse drug reactions due to ever-increasing dosages and, ultimately, leading to chronic allergic diseases. Moreover, avoidance and immune therapy are often ineffective in practice, and the surgical method often requires consistent treatment even after the surgery itself.

What's Novel about Bendu-381®?
Bendu-381® is an innovative combination of 3 herbal extracts. Unlike other allergy medications that use chemical compounds, Bendu-381® is derived from raw materials that are completely edible. Each of its ingredients is an anti-oxidant that inhibits the enzyme responsible for allergic reactions (5-lipoxygenase, cyclooxygenase). Bendu-381® controls the mast cell, a critical node in the pathophysiology of allergies.
Bendu-381® features:
- Excellent inhibitory effects on the enzymes that cause allergies
- Stabilizing effects on mast cells
- Relief from allergies in about 2 weeks, with most symptoms gone or reduced within 4 weeks
- Patented technology
- Recognition for 'Technological Excellence'
- Enhanced general quality of life, with remarkable effects on the constitutional symptoms and activity condition

Human Clinical Study Results*
The Bendu-381® treatment group showed improvement with all the major symptoms of allergic rhinitis, such as itchy nose, sneezing, and runny nose. Sneezing and runny nose saw significant decreases not only in severity but also in duration.
Analysis of the secondary efficacy variable, RQoL, revealed that out of the 7 categories for RQoL (daily life, sleep condition, nasal symptoms, general symptoms, activity condition, emotional condition, eye condition), the score for nasal symptom, general symptom, activity condition, and the overall average were decreased. The results for average RQoL and nasal symptoms showed decreases in the experimental group in the 4th week of administration compared to the control group, and the RQoL score for general symptoms and activity condition decreased by the 4th week in a statistically significant way.
From the standpoint of safety:
- No cases of severe adverse drug reactions or early termination of the clinical trial due to severe adverse effects
- No abnormal findings were detected
*66 subjects with perennial allergic rhinitis · Evaluation: TNSS (Total Nasal Symptom Score), RQoL (Rhinitis Quality of life), and Skin Prick test · double-blinded, placebo-controlled · 4 weeks, one group taking 800 mg/day of Bendu-381® · Conducted in accordance with the International Guideline for Clinical Trials (ICH GCP) · Published study
In-vivo and In-vitro Studies
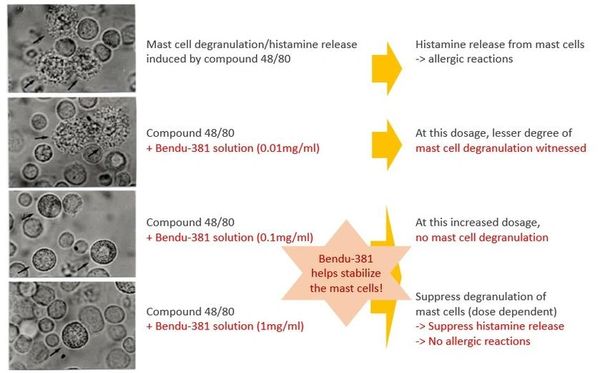
Pictured here: microscopic observations of the inhibitory effect of Bendu-381® against mast cell degranulation and histamine release.

Pictured here: Bendu-381® blocks the release of immune system chemicals that contribute to allergic reactions.

Pictured here: Animal test for atopy treatment · atopy-induced mouse → apply Bendu-381® (10mg/mouse) → measure histamine and IgE · Result: index of hypersensitivity type I is dramatically decreased and condition of skin is restored
These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure or prevent any disease. This information is furnished without warranty, representation, inducement or license of any kind. You are responsible for determining if these products are appropriate for your use.
Copyright © 2023 SunBio Corporation - All Rights Reserved.
